Dear PMI Atlanta Community,
Reflecting on our recent participation in the Latino Youth Leadership Conference hosted by Emory University fills us with immense pride and gratitude. The event, graciously made possible by the Latin American Association and the PMI Atlanta Social Impact Committee, provided an invaluable platform to share our project management journey and insights. The presentation at the Carlos Museum on November 11, 2023, featured PMI Atlanta Social Impact presenters Robert Montgomery, Cyntia Jones, and guest presenter Luis Pradillo. Each shared their unique experiences, emphasizing the blend of challenges, triumphs, and invaluable lessons encountered on their personal project management journeys.
Our interaction with high school students and college mentors within Emory's inspiring Carlos Museum setting was genuinely enriching. The exchange of ideas and knowledge amongst this diverse group was profoundly inspiring and reinforced the significance of empowering the next generation. Among the impactful insights shared were strategies to navigate challenges in project management and the importance of seizing opportunities for professional growth. We emphasized the need to encourage ambitious aspirations while underscoring the diligent work required to turn dreams into tangible realities.
As we collectively shape the future, fostering empowerment in the next generation becomes pivotal. Witnessing these future leaders' enthusiasm and potential was humbling and inspiring. We eagerly anticipate the positive impact they will create in their respective spheres.
Our heartfelt gratitude goes to the organizers for providing this exceptional platform. Through such initiatives, we collectively champion #LatinoLeadership #ProjectManagement and pave the way for a unified journey toward success. Let's celebrate the power of career journeys while emphasizing the profound significance of youth empowerment through endeavors like PMI Atlanta Social Impact.
Warm regards,
PMI Atlanta Social Impact
written by Cyntia Jones
Written by: LeDerrick Bouknight, PE, PMP
Presentation Overview:
After a brief opening and introduction, the roundtable panelists (Lee Jordan, Joe Sisto, Diem Huynh, & Dana Moore) began the discussion with the stakeholder(s) definition and expectations.
A stakeholder is a person or group that can affect a project currently in progress. The panel advised to seek to understand stakeholder’s expectations, establish a positive working relationship, complete a stakeholder assessment, and heat map at project initiation.
The next discussion topic centered around how to identify stakeholder(s). Techniques such as reviewing the project charter, obtaining referrals from other stakeholders, and discussion with the project sponsor were discussed.
The last topic of discussion was how to manage stakeholder(s) who are not supportive or seek to sabotage the project. Project managers (PM) should use their political savvy and collaborative skills to understand/address the dissenting members needs and wants. The PM should understand and adapt to the type of organizational structure (matrix) to properly leverage resources with minimal disruption.
Overall, this was an informative meeting discussing stakeholder management along with tips for doing so.
Takeaways:
o Stakeholder is a person/group that can affect a project currently in progress
o Techniques to identify stakeholders
o Techniques to manage unsupportive or disruptive stakeholders
Next Event:
Join us at the next PMI Atlanta Governance forum in February!
Register at: https://pmiatlanta.org/events/event-calendar?default_month=0
Written by:Catherine Binuya, Ed.D.
Presentation Overview: 
Presenter: Darron Bailey, MBA, PSP, is the principal founder of Construction Data and Analytics. He is a planning and schedule professional with twenty years of industry experience. His company, CDA, provides project scheduling and cost controls services in major metro areas such as Atlanta, St. Louis, Dallas, Orlando, and Nashville.
Speaking from his experiences on both public and private construction projects, Darron Bailey presented on the key steps of project planning and scheduling.
Takeaways
- Major reasons for delays—inefficient management, implementation failure, weather, design flaws, scope increase, economic changes, failure to coordinate time schedules, failures in execution. The construction industry is notoriously known for project delays. Construction Planning, if planned correctly, project schedule usually runs smoother.
- Manpower, machines, materials, and money are the top four critical factors to monitor that impact project planning and scheduling. Other factors are space, time, and tasks.
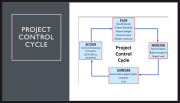
- Control Cycle is an iterative process involving four key stages: Plan, Monitor, Compare, Action. Comparisons from project updates vs. expected baseline targets calls attention if an activity slips. During Action stage, it is key to monitor variances. Projects may require setting new baseline targets.
- Project Planning and Execution are Action stages. Reporting deliverables, cost, scheduling, etc. on a bi-weekly basis allows for PMO working teams to stay abreast of the project. On projects, CDA works with cost engineers, schedulers, project managers to form a PMO group. This allows all team members to stay accountable to project completion.
- Framework, PMO, updating scheduling, Schedule Reporting (SV) – gap between baseline and current schedule represents variance, Schedule Reporting (Compression) – accelerating certain phases of a project in an attempt to get scheduling back on track, Monitoring Costs, Monitoring Cost Variance, and Critical Ratio are all key steps to project planning and scheduling.
Next Event
Join us at the next PMI Atlanta Architectural, Engineering & Construction (AEC) forum on Tuesday, December 12, 2023.
Keynote Presentation: “The Stich” presented by Jack Cebe, Stitch Development Manager, Atlanta Downtown Improvement District.
Register at:https://pmiatlanta.org/events/event-calendar
Event Pictures
“Project Management & Beyond: A Peak Into the Future": October Joint Special Interest Forums Summary
Written by: Alex Leonard, PMP
Presentation Overview
AI
Doug Ware is a Systems builder with over 30 years in the technology industry. He noted that as a kid, he was always very interested in AI. He explained about emerging technologies in AI that generate answers word-by-word. He also noted that there is a large misconception that AI can think and do things by itself, where as they actually need to be triggered by a person.
Oliver Yarbrough is a Project Management Trainer and works with AI in relation to project management. He also hosts the podcast “Not Your Father’s AI Show”. Oliver emphasized the importance of using automation to optimize workflows and minimize busy work.
HR Recruiting
Amy Chestnut is the director of HR at Rare Disease Research, LLC. Amy’s organization helps provide many services including talent acquisition, Pediatric Trials and Research. In the forum, Amy discussed the types of skills, experiences, and personalities that she might look for in clinical project management. Some of these skills include flexibility, being resourceful, and having a passion for healthcare.
Brett Horsely, with Legent Finance & Accounting, has a background in sales, marketing, and recruiting. He helps fill roles for Contract, Contract for Hire, and Full Time. Brett also hosts a podcast called “Straight From the Horsely’s Mouth” discussing tips on hiring, resumes, job seeking, and employee retention. Brett explained that we have the most diverse workplace, generationally, that the world has ever seen. He noted that the commonality between these generations is that everyone wants the same things – they just prioritize them differently.
Dana Neiger is the Co-Founder for HIVE Talent Acquisition Firm in Sandy Springs. HIVE can help in a number of areas such as fractional HR leadership and establishing HR compliance for small-to-medium business. Dana explained a few things to do when looking for a new job: 1) Track job you apply for in a spreadsheet. 2) Push the bar, but don’t put yourself in despair. 3) Understand your worth.
"DEI" | "ESG"
Solama Acolatse-Narnor, with a background in architecture, is a sustainability consultant with The Dragon Group. Solama emphasizes the need for sustainability as it humanizes the DEI & ESG Industries. She believes that performance comes from areas other than skill, such as environment, emotional safety, etc.
Dr. April Ripley, with Permier Image, is a trainer focusing on intercultural awareness. She works with clients around the world helping them bridge the gap between cultures and backgrounds in the workplace. April charged the audience to challenge assumptions and misinformation in the workplace.
Dr. Frank Lee Harper, Jr. is the Vice Chancellor at Cambridge Corporate University. Throughout the course of his career, he has had the opportunity to utilize project teams to study the effectiveness of the ways teams communicate and collaborate with each other. He discussed the importance of being a “Strategic Hustler”, or an innovative futurist.
Next Event
Join us at our final Forum of 2023, PMI Atlanta AEC Forum on Tuesday, Dec 12, 2023.
Keynote Presentation: Learn about “THE STITCH” A VERY IMPORTANT PROJECT FOR THE CITY OF ATLANTA. Presented by Jack Cebe
Register at: https://pmiatlanta.org/events/event-list
Written by: Tana Glassford-Samuel, MBA, PMP
The Best of Both Worlds. How Waterfall and Agile can co-exist.
Presentation Overview 
Tamara McLemore, PMP, PMI-ACP, a 20-year project management veteran and sought after speaker, was this month’s presenter. The August event was held in-person at Matrix, the Agile Forum’s Sponsor. Tamara spoke to a packed room. The session was engaging and thought-provoking. Waterfall with Agile, in any combination, can be beneficial for project managers (PMs) and offer real value to the organization.
Agile is a mindset change and has standard terms and events/ceremonies. However, the Agile nomenclature can be a blocker in a strong Waterfall culture. In some minds, Agile is a bad word and PMs may need to work around it by using different terms to achieve the same results – value-driven delivery.
Takeaways
- The Benefits of Agile
- Faster to Market
- We are not in the microwave era; We are in the air fryer era!
- Flexibility
- Instant Feedback
- Save Millions of Dollars and time
- Faster to Market
- The Four Values of the Agile Manifesto
- Individuals and interactions over processes and tools
- Emphasizes the need to deliver
- Working software over comprehensive documentation
- Do not show elaborate documents, demo the product
- Customer collaboration over contract negotiation
- Be flexible and accommodating
- Responding to change over following a plan
- Change is inevitable
- Individuals and interactions over processes and tools
- Incorporate Agile components in Waterfall projects
- Minimal Viable Product (MVP)
- Allows for early return on investment
- Definition of Done
- What does success looks like
- T-Shirt Sizing
- Relative estimation of the work
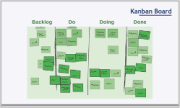
- Kanban Board
- Tells the true status/state of play
- Minimal Viable Product (MVP)
Next Event
Join us at the next PMI Atlanta Chapter Agile forum on Tuesday, September 19, 2023
Keynote Presentation: "Artificial Intelligence for Agile Project Managers: Are You Ready" by Scott Ambler, Author & Agile Data Coach, Ambysoft, Inc.
Register at www.pmiatlanta.org/events/event-calendar